PROTAC Targeted Protein Degradation(TPD)Platform
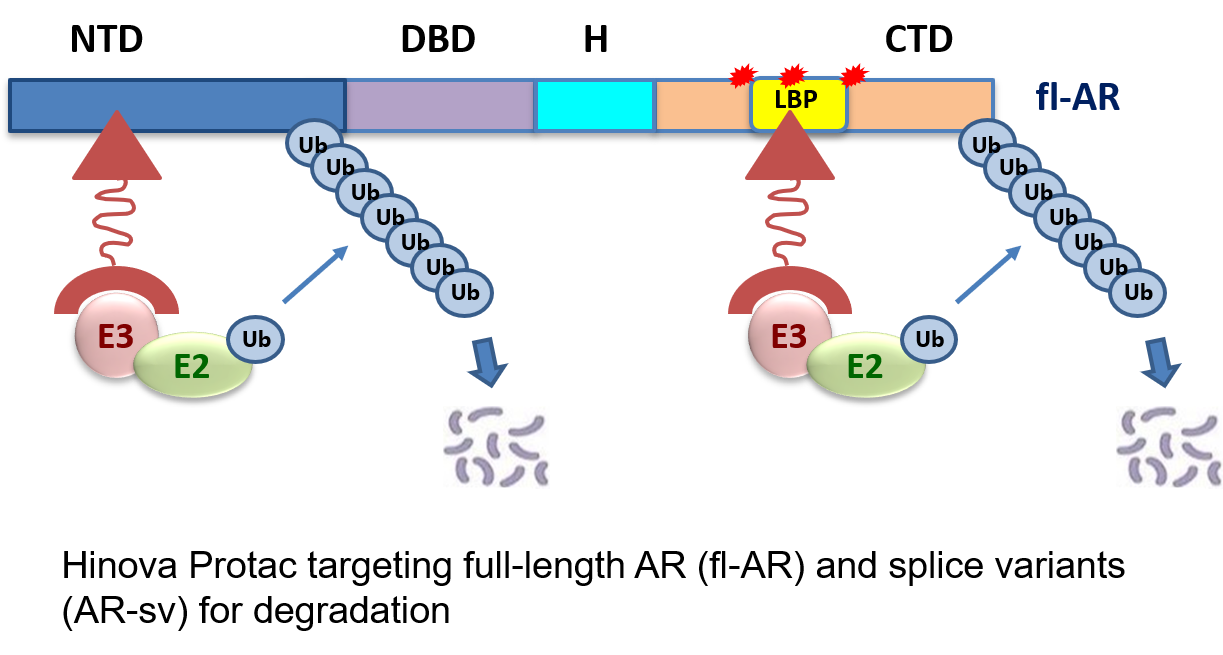
Competitive Advantages of Hinova PROTAC Platform
Deuterated Drug R&D Platform
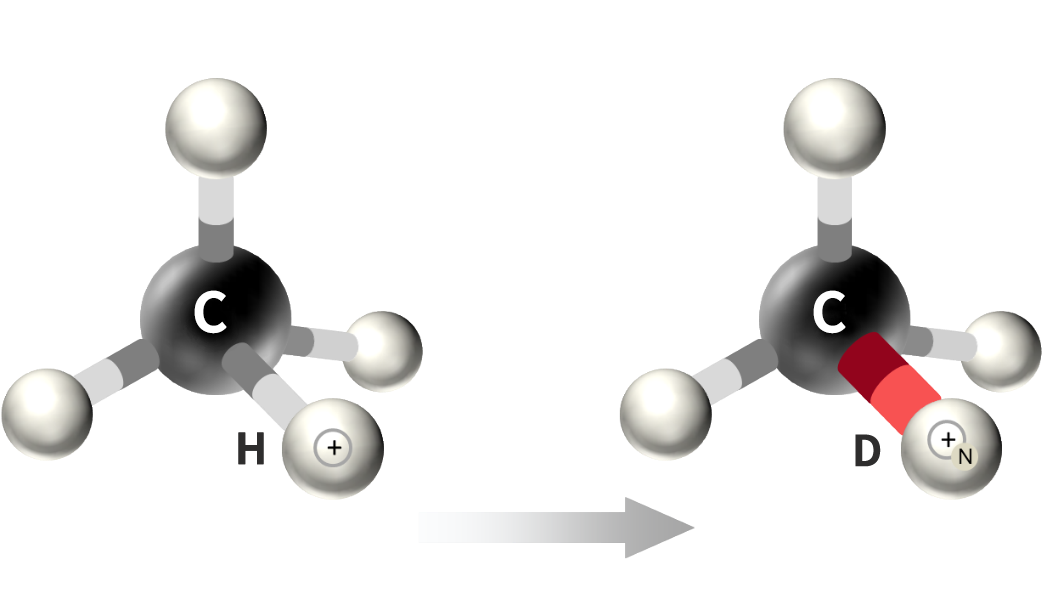
Competitive Advantages of Hinova Deuteration Technology Platform
Targeted Drug Identification and Validation Platfo
During drug discovery, proper target identification is the most important step.
A good target is supposed to be safe, effective, druggable and meets medical and market needs.
Our R&D team is comprised of accomplished experts in biology, pharmaceutical chemistry, pharmacokinetics, pharmacology and clinical operation, who established a solid foundation for the identification and validation of new drug targets, and the development of pipelines.
What We Can Do on Targeted Drug Discovery and Validation Platform
Lead Compound Optimization and Screening Platform